9.1. All Ceramic Restorations
Ceramics show low fracture resistance when compared with metals. Metal-ceramic systems unify the aesthetical properties of ceramics and the extreme mechanical properties of metals. However, metals may cause problems such as aesthetics, allergies, gingival coloration and release of metal ions into the gingival tissue and fluid. Correspondingly, the ceramic systems used without metal infrastructure were successfully implemented at the beginning, in the anterior region and as material of single crown, however, they found limited application area in premolar and molar regions. With the development of these materials, it has been possible to use them as posterior-long membered fixed partial restorations and implant superstructures. These systems are examined under the name of full ceramics with different structure and production methods.
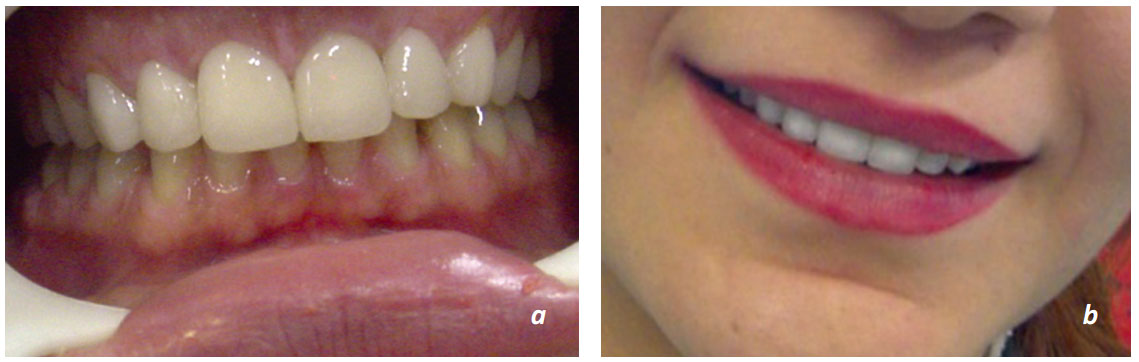 Figure 9.1 a. b. Full ceramic systems were successfully implemented as a restoration material of single crowns and in the anterior region.
Figure 9.1 a. b. Full ceramic systems were successfully implemented as a restoration material of single crowns and in the anterior region.
Classification
Ceramics can generally be classified according to their microstructure or processing techniques.
Microstructural Classification
At the microstructural level, they are examined in subgroups according to glass-crystalline ratios.
- 1. Glass based systems (mainly silica)
- 2. Glass based, filled systems /leucite or lithium disilicate as crystalline phase (mainly silica)
- 3. Crystalline-based, glass-filled systems (mainly alumina)
- 4. Polycrystalline solids (alumina and zirconium)
Glass-based systems comprise alumina in various amounts, silicon dioxide, also known as silica or quartz. Aluminosilicates which are found in nature and contain different amounts of potassium and sodium are also known as feldspar and they are modified in various ways to form the glass used in dentistry. Synthetic aluminosilicate glass forms are also fabricated for dental ceramics.
Systems with glass-based fillers have glass-crystalline with a very large ratio and crystal type. As a result of this diversity, they are divided into three subgroups. The glass composition is basically identical to the pure glass category.
1. Feldspathic glass containing low and medium levels of leucite: Bu These materials are also called "feldspathic ceramics".
2. Glass containing high leucite (approx. 50%): The glass phase is an aluminosilicate glass. These materials have been developed in both powder/liquid, processable and compressible forms.
3. Lithium-disilicate glass ceramic: It is a glass ceramic type which is introduced as IPS Empress® II (now called IPS e.max®) and obtained by adding lithium oxide to the aluminosilicate glass.
The difference between them is the addition of varying amounts of different crystal types to the glass matrix. These crystal types are leucite, lithium disicilate or fluoroapatite.
Feldspathic glass ceramics reinforced with leucite, aluminosilicates found in nature and containing different amounts of potassium and sodium are known as feldspar.
Ceramics containing high quantities (35% by volume) of leucite are called pressed glass ceramics. The main component of this ceramics is feldspathic porcelain containing 63% SiO2, 19% Al203, 11% K2O, 4% Na2O and trace amounts of other oxides. Leucite crystals are added to the aluminium oxide.
This material is produced by filling the prepared mold which is known as pressing with heat and made by casting, with plasticized ceramic to prevent sintering and subsequent pore formation. Leucite crystallization provides the ceramic the dispersion hardening, a process which is used to stop the fracture propagation of dispersed phase of a different material (such as alumina, leucite, zirconia). Because, it is more difficult that these crystalline phases penetrate through fractures.
A glass ceramic (IPS Empress II*) based on SiO2-Li2O system has been developed in order to extend the use of lithium disilicate glass ceramics, ceramic restorations bonded with resin and by any means, to use them for bridges. Crystalline fill particles have been added to increase the strength, thermal expansion and contraction behaviour of the ceramics. Among the other fillers adding types, there are glass particles with high melting points which are stable at the firing temperature of the ceramic. The crystalline phase created, is a lithium disilicate (Li2Si2O5) and forms 70% of the glass ceramic volume. Lithium disilicate has an unusual microstructure. This is ideal in terms of durability.
There is also a second crystalline phase consisting of a much lower volume of lithium orthophosphate (Li3PO4). The mechanical properties of this glass ceramic are much better than those of leucite glass ceramics with a bending resistance in the range of 350-450 MPa and a fracture resistance of about three times that of the leucite glass ceramic. It is claimed that the glass ceramic is crystalline in a high level depending on the optical compatibility between the glassy matrix and the crystalline phase and which minimizes spreading of the light inside the material.
The processing way is the same as the heat pressing described above, except that the processing temperature at 920°C is lower than that of leucite glass ceramic. The particle size of the lithium metasilicate crystals diverges from 0.2 µm to 2 by providing a bending resistance of 130 MPa to this material. This is similar to the other milling-ready leucite-reinforced CAD/CAM (ProCAD)* blocks and feldspathic CAD/CAM blocks (Vitabloc Mark II)**.
Glass filler, crystalline based systems were presented as glass infiltrated, partially sintered alumina in 1988 and marketed under the name of In-Ceram. The system was developed as an alternative of conventional metal ceramics and achieved important clinical success.
The infiltrated ceramics are produced by a process called slip casting which contains condensation of an aqueous ceramic on the refractory day. Glass is infiltrated to this kiln-dried porous infrastructure at high temperatures. The materials processed in this way exhibit less porosity, less defect due to the process, more resistance and more hardness than conventional feldspathic porcelains.
Then, this glass infiltrated infrastructure is coated with a feldspathic ceramic. They have perfect translucency and aesthetic properties. After all, physical properties are inadequate. The Vita In-Ceram* slip casting system uses three different materials to provide a good fit between resistance and aesthetic.
In-Ceram spinel**: Spinel (MgAl2O4) is a natural mineral that is commonly found in limestone and dolomite together. It is important for dentistry because of its extremely high melting point (2135°C) together with its high strength. Spinel is chemically inert and has low electrical and thermal conductivity. In addition to this, most importantly, it exhibits unequalled optical features. It has a medium resistance around 350 MPa and a good translucency property.
Depending on phase fraction index of the crystalline close to that of glass, In-Ceram has a translucency property over two layers compared to alumina. Glass infiltration in a vacuum environment leads to less porosity which provides this high translucency. Additionally, mostly, this translucency level may be excessive and may lead more glassy and low-valued appearance.
In-Ceram alumina**: Aluminum oxide (Al2O3) is most commonly known under the term of corundum. As a result of the homogeneous infrastructure of fine Al2O3 particles filled with a special glass gaps, the tensile strength is significantly higher than that of the other full ceramic systems. Aluminum oxide, with a weight ratio of 10-20%, is a feldspathic component which is starting material for metal-ceramic veneer materials. The ceramic materials for jaket crowns’ infrastructures are enhanced with aluminium oxide crystals with a particle size of 10-30 µm to 60% by weight for increasing stability. In-Ceram alumina has a resistance of around 500 MPa and a weak translucency.
In-Ceram zirconia**: Zirconia system uses a mixture of zirconium oxide and aluminum oxide to execute a significant increase in the bending strength of the core structure. The aluminum oxide forms two out of three of the crystalline structure. The remaining crystalline structure consists of tetragonal zirconium oxide. The glass phase ratio forms 20-25% of the total structure. This provides high resistance as already seen in In-Ceram alumina. This increase depends on zirconium oxide particles which protect the alumina against the crack propagation. It has a resistance of approximately 700 MPa and a very weak translucency.
Polycrystalline solids, solid-sintered and monophase ceramics are materials form by direct sintering of crystals without any matrix interference to form a dense, airless, glassless, polycrystalline structure. There are different processing techniques that allow the production of solid sintered aluminous oxide or zirconia-oxide infrastructure.
Zirconia is a natural mineral which is also called baddeleyite. This mineral contains 80-90% zirconium oxide. It mostly contains TiO2, SiO2 and Fe2O3. This oxide has three different crystal structures; it is in monoclinic at room temperature, tetragonal at about 1200°C and cubic at 2370°C. Zirconium oxide is transformed from one crystalline state to another during the kiln-drying process. At kiln-drying temperature, zirconia is tetragonal and at room temperature it is monoclinic by covering nearly 4.4% more volume than a unit monoclinic tetragonal.
By the end of the 1980s, the tetragonal form was stabilized at room temperature by adding a small amount (3-8% by mass) of calcium and yttrium or cerium. Although it is stabilized at room temperature, the tetragonal form is "semi-balanced" and this means that there is the trapped energy that will bring the material. A highly localized stress before a crack indicate propagation is enough to trigger the transformation of ceramic grains in the proximity of this crack tip. In this case, the volume increase of 4.4% is advantageous particularly to narrow the closed crack, ie. the transformation reduces the severity of local stress.
Two major developments have made polycrystalline ceramics practical for fixed dentures:
1. The presence of highly controlled starting powders and
2. Computer application to ceramic processing process.
*Ivoclar Vivadent / Lichtenstein
**VITA Zahnfabrik, Bad Säckingen/ Germany

